Accuracy and Precision Lab Chemistry Answers
Accuracy and Precision of Different Types of Glassware Lab PreLab. The precision of a set of measurements can be determined by calculating the standard deviation for a.
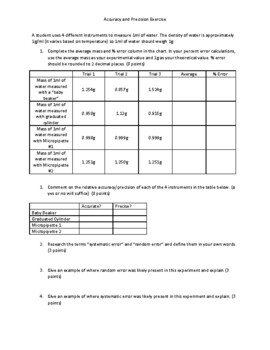
Accuracy Precision Reading Lab Equipment And Significant Digits Virtual Lab
Refers to the reproducibility of a series of measurements.

. Be able to distinguish between the accuracy and precision of estimates made of the measure of several different quantities. Step 1 Take the difference between accepted value and experimental value. Precision marks of an object.
Beaker Erlenmeyer Flask Graduated Cylinder Reusable Plastic Cups Triple Beam Balance Procedure 1. Step 3 Divide the answer obtained from step 2 by the accepted value. A zero end or zero in decimal place only significant.
Is actually that will awesome. Place 4 dots on each target with the appropriate level of accuracy and precision. Define Accuracy and Precision 2.
Step 4 Multiply the answer got in step 3 by 100. Accuracy and Precision of Laboratory Glassware. I had to round my answer to two sig figs giving me the answer 42 grams.
Accuracy Precision and Error Lab Accuracy is the closeness of a measurement to the true value of what is being measured. The student will be able to distinguish between the accuracy and precision of estimates made of the measure of several different quantities. I had to round my answer to three sig figs giving me the answer 659 grams.
Step 2 Take the absolute value of step 1. Record the new water level in the table. Choose an answer and hit next.
Measure and record the mass of the three plastic cups 2. For Devices C B A. Accuracy refers to the proximity of a value measured at a standard or known value.
Record this volume in the data table for part B. 253460 04223333 42g The least number of sig figs in my given measurements is two sig figs. If you believe consequently Il m.
Precision A term used to describe how close repeated measurements are to each other. We tried to locate some good of Accuracy and Precision Chemistry Worksheet Answers with 1 2 Problem Set 1 Significant Figures Accuracy and Precision image. Accuracy Accuracy is a combination of trueness and precision.
Accuracy And Precision Worksheet Answers. Measure and record the mass of the cylinder and water as accuratelyas possible. To assess accuracy you need to know the true or theoretical value from some source.
Numbers that are significant zero-zero digits are always significant. Students calculate density and are introduced to its dependence on temperature. There is a logical relationship between these six terms as shown in Figure 1 and Table 1.
How close a measurement is to its actual value determined by the percent error formula. Mark each set of numbers as having a high or low accuracy and precision. Accuracy How close a measurement is to some accepted true value.
Determining the Density of Water During the semester in the general chemistry lab you will come into contact with various pieces of laboratory glassware. In this lab exercise students are introduced to various measurement tools ruler graduated cylinder triple beam balance and taught how to use them and correctly record data correct number of significant figures. Chemistry Name Date Class Accuracy and Precision Lab Accuracyhow close a measurement is to an accepted true value Precisiona tern used to describe how close repeated measurements are to each other Objective.
Write and analogy to remember each of the vocabulary words Materials. Delightful to help my own blog in this particular moment I will explain to you with regards to Accuracy And Precision Worksheet Answers. Why dont you consider photograph previously mentioned.
One way of estimating precision is to use the average deviation finding how far each individual measurement x is from the average value and then taking the average eq 1 n the number of data points you have. Up to 24 cash back Chemistry. Add enough dry aluminum pellets so that the water level rises at least 5 mL.
It refers to how many individual measurements agree with the correct or true value. Mass Data of Sample Trial 1 Trial 2 Trial 3 Trial 4 Student A 143 g 152 g 147 g 142 g Student B 143 g 140 g 146 g 144 g Student C 154 g 156 g 158 g 150 g Student D 086 g 124 g 152 g 142 g 12. Divide the mass of the solid by the volume of the solid calculated above Trial 1 42313499184626 Trial 2.
How many pieces of data are grouped around a similar reading after repeated trials. Observed Value-Actual Value Actual Value x 100. Good accuracy requires good trueness and good precision.
The accuracy of an experimental value is best determined by the average value of multiple measurements where x i represents a measurement and n is the number of measurements. Add about 10 mL of water to the 25 mL graduated cylinder. 1 the precision in the measurements improves from the Devices C to B to A as indicated by the increasing trend in the SD.
The 2 is underlined in the unrounded answer because based on the addition rule when the answer. Accuracy And Precision Worksheet Answers. To generate all the multiples of 5 between 5 and.
Step 5 Add percentage symbol to indicate the answer in percentage. Density of Regular-Shaped Solid Calculate the density of the irregular-shaped solid for each trial. Balance Lab Worksheet Part III.
0107 Accuracy and Precision. Accuracy and precision lab chemistry answers Accuracy and precision. You will receive your score and answers at.
For example if a weight of 32 kg is obtained in the laboratory for a certain substance but the actual or known weight is 10 kg then the measure is not accurate. Accuracy Precision Worksheet Accuracy. Precision can also refer to how many digits are you able to measure with a piece of equipment sometimes referred to as numerical or arithmetic precision.
Laboratory techniques of the analyst improve. Accuracy and precision worksheet answers. Object measured is 10 meter long.
If you are told the length of. Definitions Comparisons - Quiz Worksheet. A measure of how intimately individual measurements agree with each other.
Accuracy is a measure of how close the measured value is to the true value. Six terms related to accuracy are. Precision is the reproducibility of a set of measurements taken under the same conditions.
Precision Trueness Accuracy Random Error Systematic Error and Uncertainty. Calculations of accuracy percent error and precision average. Four students each measured the mass of one 143 g sample four.
Zero between two digits zero is significant.

Significant Figures Accuracy Precision Lab Activity By Volkamania

The Purpose Of This Lab Was To Differentiate Between Accuracy And Precision By Calculating The Mass Per Millimeter Or The Density Of The Unknown Liquid Using Two Different Methods Gcse Science
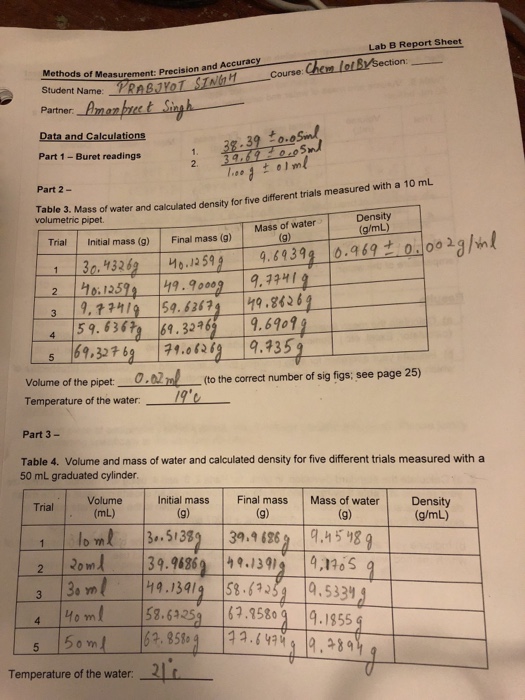
Solved Lab B Report Sheet S Of Measurement Precision And Chegg Com

Solved Experiment 3 Data Chem 0110 Measurements Chegg Com

Worksheet Accuracy And Precision Final Science Measurement And Uncertainty Accuracy And Precision Name Period Date Accuracy And Course Hero
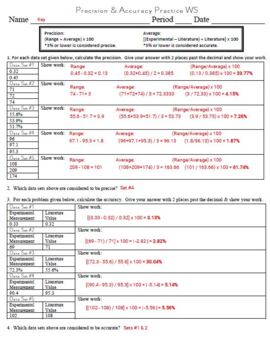
Calculating Accuracy Precision Ws For General Chemistry By Chem Queen

Lab 1 Experiment 1 Accuracy And Precision Oer Part 1 Pdf Lab 1 Accuracy And Precision How Good Of A Shot Are You Procedure Part 1 1 Find Course Hero

Quiz Worksheet Accuracy Vs Precision In Chemistry Study Com

Precision Accuracy Percent Error Notes

Solved Methods Of Measurement Precision And Accuracy Lab B Chegg Com
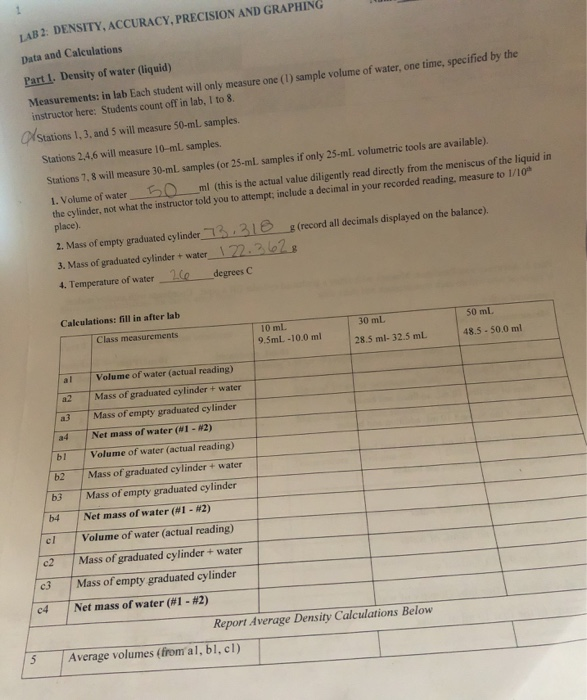
Solved 1 Lab 2 Density Accuracy Precision And Graphing Chegg Com
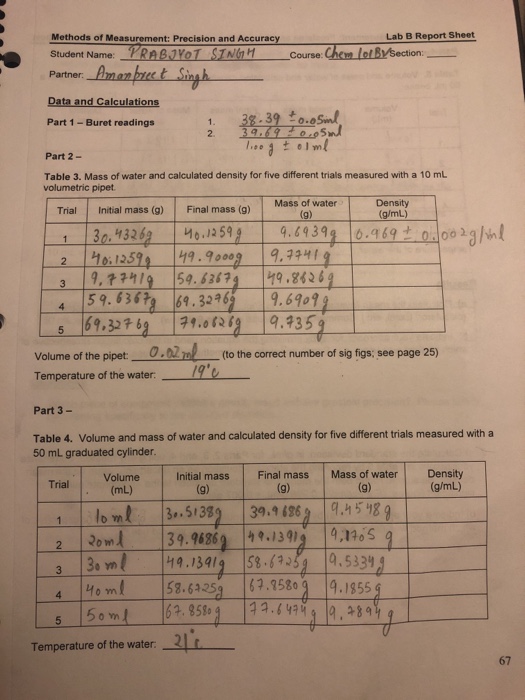
Solved Methods Of Measurement Precision And Accuracy Lab B Chegg Com

Accuracy And Precision 1 Section 4 Data Analysis Accuracy And Precision Graham Mueller Hour 8 1 Course Hero

Solved Lab B Report Sheet Methods Of M Measurement Chegg Com

Ps 03 2019 Key Key Chem 105 General College Chemistry Byu Studocu



Comments
Post a Comment